
If you have any queries related to this page, reach us through the comment section below and we will get back to you as soon as possible. We hope this article on Charged Particles of Matter is helpful to you. They are also known as subatomic particles. Examples of Charged Particles in Matter?Īns: In an atom, there are three fundamental charged particles: Electrons, Protons, and Neutrons. The name electron was adopted for these particles by the scientific community, mainly due to the advocation by G. The hydrogen ion is an example of a proton. He further showed that the negatively charged particles produced by radioactive materials, by heated materials and by illuminated materials were universal. The charge of a proton is equal to the charge of an electron in magnitude, but the charge is positive. What do you call the positively charged particle?Īns: A positively charged particle in an atom is known as a proton. The implication of this is that negatively charged nutrients such as nitrates, sulphate and chlorides are vulnerable to leaching down the soil profile. On the other hand, negatively charged soil particles repel anions (negatively charged ions). Mostly, an imbalance of charged particles, i.e., electrons or protons in an atom, causes a particle to be charged. The cations held by the soil particles are called exchangeable cations. We review their content and use your feedback to keep the quality high. Who are the experts Experts are tested by Chegg as specialists in their subject area. What causes a particle to be charged?Īns: When particles are excited by imparting energy, they become charged. This problem has been solved Negatively charged particles of radiation emitted from the decay of radioactive substances are known as. In both cases, when a particle is charged, electrons are transferred.
What happens when a particle is charged?Īns: When a particle is charged, it becomes negatively charged on gaining electrons or becomes positively charged on releasing electrons. FAQs on Charged Particles in MatterĪns: A charged particle is called an ion or an atom that consists of an unbalanced number of electrons or protons, making it a negatively charged or positively charged atom.Īns:An atom contains three types of charged subatomic particles: (i) Negatively charged electron, (ii) Positively charged proton, and (iii) uncharged neutron.

Electrons are the lightest subatomic particles, and the maximum mass of an atom is confined to its nucleus, where protons and neutrons are present. By the \(1900\) and that of a proton is almost like an electron numerically but positively charged. In \(1886\), scientists noticed some radiations coming out of the atom, and this was the first step towards believing the fact that there was something inside the atom. Earlier, scientists believed that the atom is the smallest particle that can exist, but with time, they discovered that there is something inside the atom. suggested earlier that electrons could be defined as those small negatively charged particles that orbit the nuclei of atoms, and phlogiston as that.
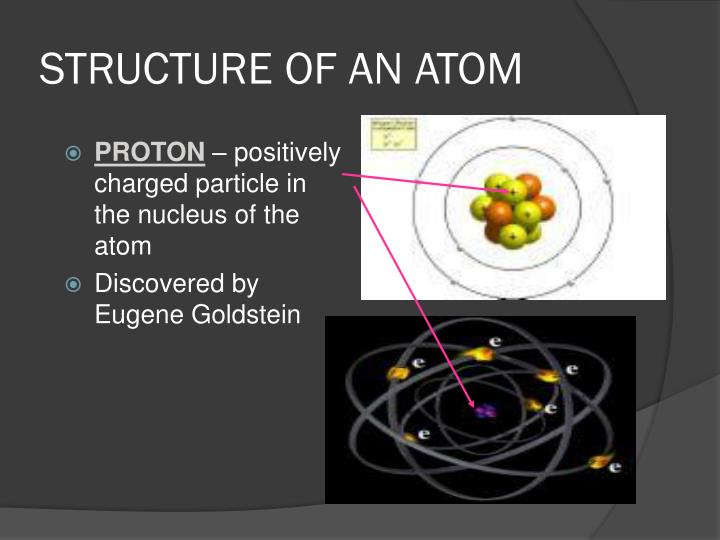
All matter is made up of the smallest unit known as the “Atom”. Charged Particles in Matter: Matter is defined as any substance that has mass and occupies space.


 0 kommentar(er)
0 kommentar(er)
